BATTERY OPERATIONS
Batteries
Internal Resistance
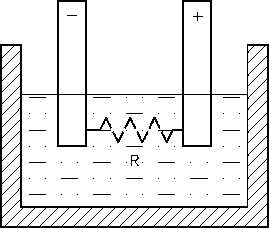
Figure 9 Internal Resistance in a Chemical Cell
Internal resistance in a chemical cell is due
mainly to the resistance of the electrolyte between
electrodes (Figure 9).
Any current in the battery must flow through the
internal resistance. The internal resistance is in
series with the voltage of the battery, causing an
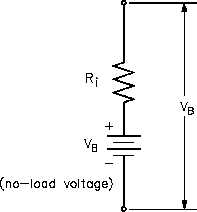
internal voltage drop (Figure 10).
With no current flow, the voltage drop is zero;
thus, the full battery voltage is developed across
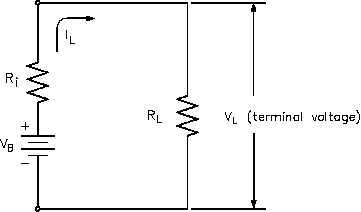
the output terminals (VB). If a load is placed on
the battery, load resistance (RL) is in series with
internal resistance (Ri).
When current flows in the circuit (IL), the internal voltage drop (ILRi) drops the terminal voltage
Figure 10 Internal Voltage Drop
of the battery as shown in Equation (4-3). Thus, internal resistance reduces both the current and
voltage available to the load.
VL = VB - ILRi
(4-3)
ES-04
Page 12
Rev. 0