O2
+ 4H
2H2O
Corrosion
DOE-HDBK-1015/1-93
GENERAL CORROSION
Rev. 0
CH-02
Page 13
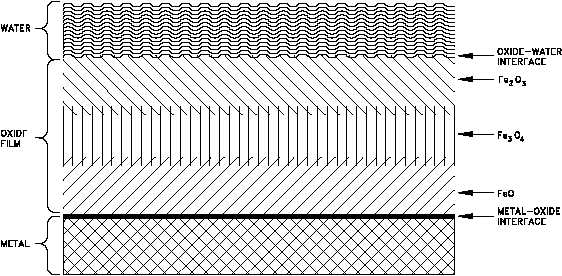
Figure 5 Simplified Schematic Diagram of Oxide Corrosion Film on the Surface of a Metal
Regardless of the exact diffusion mechanism, the oxide layer represents a barrier to continued
corrosion and tends to slow the corrosion rate. The exact effect of this layer on the corrosion
rate depends on the uniformity and tenacity of the film. If the film is loosely attached, develops
defects, or is removed, the metal surface is again exposed to the environment and corrosion
occurs more readily.
Factors Affecting General Corrosion Rate
Like most other chemical reactions, corrosion rates increase as temperature increases.
Temperature and pressure of the medium govern the solubilities of the corrosive species in the
fluid, such as oxygen (O ), carbon dioxide (CO ), chlorides, and hydroxides. A rule of thumb
2
2
is that the reaction rate doubles with a 20?F to 50?F temperature rise. This linear increase with
temperature does not continue indefinitely due, in part, to a change in the oxide film.
When water velocity is extremely high, the impact of the water tends to remove the protective
oxide layer and some of the metal under it (erosion), thus, exposing more metal to corrosion.
Water velocities of 30 to 40 ft per second are usually considered to cause erosion.
The presence of oxygen in water to which iron is exposed increases the corrosion rate. The
reason for this increase is the rapid reaction between oxygen and the polarizing layer of atomic
hydrogen absorbed on the oxide layer. The following reaction rapidly removes the polarizing
layer.
(2-11)