Reactor Water Chemistry
DOE-HDBK-1015/2-93
CHEMISTRY PARAMETERS
Rev. 0
CH-03
Page 19
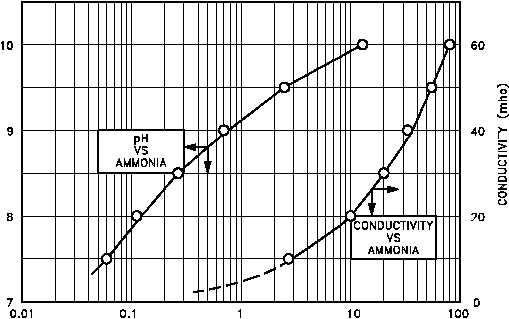
Figure 4 pH and Conductivity as a Function of NH Concentration
3
Conductivity
Conductivity of reactor facility water is measured to provide an indication of dissolved ionic
substances in the coolant. Conductivity measurements provide quantitative rather than
qualitative information because it is possible to determine the total conductivity of the ions
present, but not the specific types of ions present. Because many ions such as iron (Fe
),
+++
chromium (Cr
), copper (Cu ) and aluminum (Al
) are susceptible to forming oxides and
+++
++
+++
plating out as scale on heat transfer surfaces, reactor coolant conductivity is normally controlled
at a level as low as practicable and consistent with pH. By monitoring conductivity levels in
the reactor facility systems, the operator is able to cross check the chemistry of these systems,
thereby achieving a higher confidence level in the parameters measured.
Regardless of the operating limits specified for a given reactor facility, operating relationships
can be established between pH and conductivity levels of the coolant. Figure 4 shows a typical
relationship of the pH and conductivity of a reactor coolant system using high pH, ammonium
hydroxide chemistry control as a function of the ammonia (NH ) concentration.
3