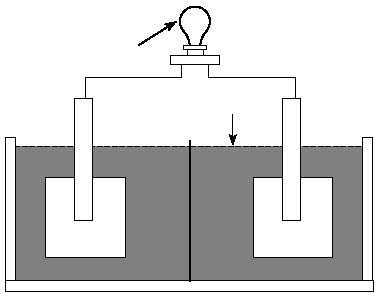
External load
Electrolyte
Porous separator
Negative
electrode
Positive
electrode
BATTERY COMPONENTS
AND OPERATION
DOE-HDBK-1084-95
Lead-Acid Storage Batteries
Batteries
Page 6
Rev. 0
Figure 1. Major components of a cell.
by cell type. Materials used as separators must allow ion transfer between the electrolyte
and electrodes. Many separators are made of a porous plastic or glass fiber material. The
above components are housed in a container commonly called a jar or container.
Cell and Battery Voltage
In order for a cell or battery to be able to deliver electrical current to an external circuit, a
potential difference must exist between the positive and negative electrodes. The potential
difference (usually measured in volts) is commonly referred to as the voltage of the cell or
battery. A single lead-acid cell can develop a maximum potential difference of about 2 V
under load. A completely discharged lead-acid cell has a potential difference of about
1.75 V, depending on the rate of discharge.
Capacity and Battery Ratings
In general terms, the capacity of a cell/battery is the amount of charge available expressed in
ampere-hours (Ah). An ampere is the unit of measurement used for electrical current and is
defined as a coulomb of charge passing through an electrical conductor in one second. The
capacity of a cell or battery is related to the quantity of active materials in it, and the amount
of electrolyte and the surface area of the plates. The capacity of a battery/cell is measured
by discharging at a constant current until it reaches its terminal voltage (usually about 1.75
volts). This is usually done at a constant temperature, under standard conditions of 25ºC