ATOM AND ITS FORCES
Basic Electrical Theory
Due to the force of its electrostatic field, these electrical charges have the ability to do work by
moving another charged particle by attraction and/or repulsion. This ability to do work is called
"potential"; therefore, if one charge is different from another, there is a potential difference
between them. The sum of the potential differences of all charged particles in the electrostatic
field is referred to as electromotive force (EMF).
The basic unit of measure of potential difference is the "volt." The symbol for potential
difference is "V," indicating the ability to do the work of forcing electrons to move. Because
the volt unit is used, potential difference is also called "voltage." The unit volt will be covered
in greater detail in the next chapter.
Free Electrons
Electrons are in rapid motion around the nucleus. While the electrostatic force is trying to pull
the nucleus and the electron together, the electron is in motion and trying to pull away. These
two effects balance, keeping the electron in orbit. The electrons in an atom exist in different
energy levels. The energy level of an electron is proportional to its distance from the nucleus.
Higher energy level electrons exist in orbits, or shells, that are farther away from the nucleus.
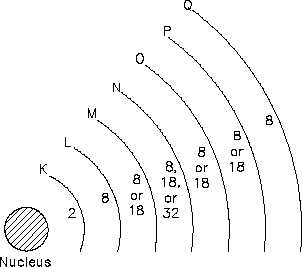
These shells nest inside one another and surround the nucleus. The nucleus is the center of all
the shells. The shells are lettered beginning with the shell nearest the nucleus: K, L, M, N, O,
P, and Q. Each shell has a maximum number of electrons it can hold. For example, the K shell
will hold a maximum of two electrons and the L shell will hold a maximum of eight electrons.
As shown in Figure 8, each shell has a specific number of electrons that it will hold for a
particular atom.
Figure 8 Energy Shells and Electron Quota
ES-01
Page 6
Rev. 0