Plant Materials
DOE-HDBK-1017/2-93
EFFECT DUE TO NEUTRON CAPTURE
Swelling can also result from gases produced in materials, such as helium formed by (n,a)
reactions and other gaseous impurities present in the metals. These traces of gas increase the
concentration of voids formed upon exposure to radiation. For example, the (n,a) and (n,2n)
reactions between fast neutrons and beryllium form helium and tritium gases that create swelling.
Under certain conditions, embrittlement can be enhanced by the presence of the helium bubbles
(helium embrittlement). The accepted view is that this embrittlement is the result of stress-
induced growth of helium gas bubbles at the grain boundaries. The bubbles eventually link up
and cause intergranular failure.
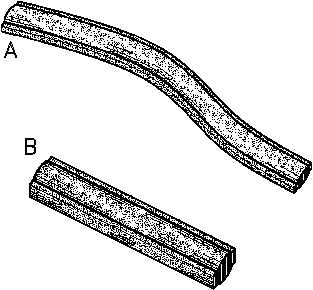
Fissionable metals suffer from radiation
Figure 6 (a) Growth of Uranium Rod;
(b) Uranium Rod Size Dummy
damage in a manner similar to that
encountered
in
structural
alloys.
Additional problems are introduced by
the high energy fission fragments and the
heavy gases xenon and krypton, which
appear among the fission products. Two
fragments that share 167 MeV of kinetic
energy, in inverse proportion to their
atomic masses, are produced from each
fission. Each fragment will have a range
of several hundred angstroms as it
produces a displacement spike. A core
of vacancies is surrounded by a shell of
interstitials,
producing
growth
and
distortion. Figure 6 shows the growth in
a uranium rod upon irradiation.
The gas formation produces eventual
swelling of the fuel and may place the
cladding under considerable pressure as
well. One of the major challenges in alloying metallic uranium is the attainment of better
stability under irradiation. Small additions of zirconium have shown marked improvement in
reducing growth and distortion.
The physical effects of ionizing radiation in metals is a uniform heating of the metal. Ions are
produced by the passage of gamma rays or charged particles through the metal, causing
sufficient electrical interaction to remove an external (or orbital) electron from the atom. Metals
with shared electrons, which are relatively free to wander through the crystal lattice, are effected
very little by ionization.
Rev. 0
Page 43
MS-05