Thermodynamics
COMPRESSION PROCESSES
The ideal gas law is utilized by engineers working with gases because it is simple to use and
approximates real gas behavior. Most physical conditions of gases used by man fit the above
description. Perhaps the most common use of gas behavior studied by engineers is that of the
compression process using ideal gas approximations. Such a compression process may occur
at constant temperature (pV = constant), constant volume, or adiabatic (no heat transfer).
Whatever the process, the amount of work that results from it depends upon the process, as
brought out in the discussion on the First Law of Thermodynamics. The compression process
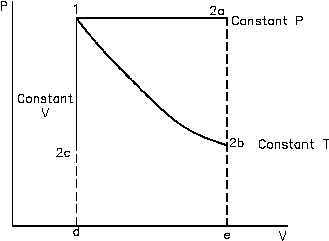
using ideal gas considerations results in work performed on the system and is essentially the area
under a P-V curve. As can be seen in Figure 40, different amounts of work result from different
ideal gas processes such as constant temperature and constant pressure.
Figure 40 Pressure-Volume Diagram
Fluid
A fluid is any substance that conforms to the shape of its container. It may be either a liquid
or a gas.
Compressibility of Fluids
Usually a fluid may be considered incompressible when the velocity of the fluid is greater than
one-third of the speed of sound for the fluid, or if the fluid is a liquid. The treatment of a fluid
that is considered incompressible is easy because the density is assumed to be constant, giving
a simple relationship for the state of the substance. The variation of density of the fluid with
changes in pressure is the primary factor considered in deciding whether a fluid is
incompressible.
Rev. 0
Page 99
HT-01