COMPRESSION PROCESSES
Thermodynamics
Ideal Gas Law
By combining the results of Charles' and Boyle's experiments, the relationship
(1-42)
Pv
T
constant
may be obtained. The constant in the above equation is called the ideal gas constant and is
designated by R; thus the ideal gas equation becomes
Pv = RT
(1-43)
where the pressure and temperature are absolute values. The values of the ideal gas constant
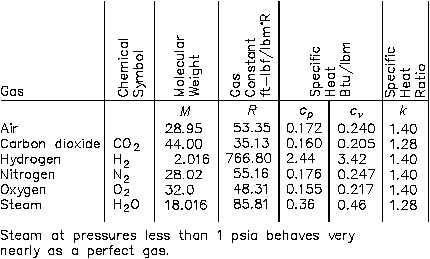
(R) for several of the more common gases are given in Figure 39.
Figure 39 Ideal Gas Constant Values
The individual gas constant (R) may be obtained by dividing the universal gas constant (Ro) by
the molecular weight (MW) of the gas,
. The units of R must always be consistent
R
Ro
MW
with the units of pressure, temperature, and volume used in the gas equation. No real gases
follow the ideal gas law or equation completely. At temperatures near a gases boiling point,
increases in pressure will cause condensation to take place and drastic decreases in volume. At
very high pressures, the intermolecular forces of a gas are significant. However, most gases are
in approximate agreement at pressures and temperatures above their boiling point.
HT-01
Page 98
Rev. 0