Thermodynamics
FIRST LAW OF THERMODYNAMICS
This
example
demonstrates
that
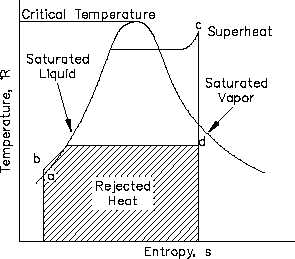
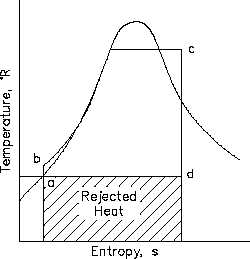
Figure 19 T-s Diagram with Rankine Cycles
potential and kinetic energy terms are
insignificant for a turbine, since the
Dpe and Dke values are less than 1
Btu/lbm.
When the system (the fluid being
studied)
changes
its
properties
(temperature, pressure, volume) from
one value to another as a consequence
of work or heat or internal energy
exchange, then it is said that the fluid
has gone through a "process."
In
some processes, the relationships
between pressure, temperature, and
volume are specified as the fluid goes
from one thermodynamic state to
another. The most common processes
are those in which the temperature,
pressure, or volume is held constant
during the process. These would be
classified as isothermal, isobaric, or
isovolumetric processes, respectively.
Iso means "constant or one." If the
fluid passes through various processes
and then eventually returns to the
same state it began with, the system is
said to have undergone a cyclic
process. One such cyclic process used
is the Rankine cycle, two examples of
which are shown in Figure 19.
The processes that comprise the cycle
are described below.
ab:
Liquid is compressed
with no change in
entropy (by ideal pump).
bc:
Constant pressure transfer of heat in the boiler. Heat is added to the compressed
liquid, two-phase, and superheat states.
cd:
Constant entropy expansion with shaft work output (in ideal turbine).
da:
Constant pressure transfer of heat in the sink. Unavailable heat is rejected to the
heat sink (condenser).
Rev. 0
Page 61
HT-01