Properties of Metals
DOE-HDBK-1017/1-93
HYDROGEN EMBRITTLEMENT
If the metal is under a high tensile stress, brittle
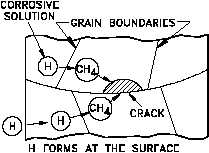
Figure 10 Hydrogen Embrittlement
failure can occur. At normal room temperatures, the
hydrogen atoms are absorbed into the metal lattice
and diffused through the grains, tending to gather at
inclusions or other lattice defects. If stress induces
cracking under these conditions, the path is
transgranular. At high temperatures, the absorbed
hydrogen tends to gather in the grain boundaries and
stress-induced cracking is then intergranular. The
cracking of martensitic and precipitation hardened
steel alloys is believed to be a form of hydrogen
stress corrosion cracking that results from the entry
into the metal of a portion of the atomic hydrogen that is produced in the following corrosion
reaction.
3 Fe + 4 H2O Fe3O4 + 4 H2
Hydrogen embrittlement is not a permanent condition. If cracking does not occur and the
environmental conditions are changed so that no hydrogen is generated on the surface of the
metal, the hydrogen can rediffuse from the steel, so that ductility is restored.
To address the problem of hydrogen embrittlement, emphasis is placed on controlling the amount
of residual hydrogen in steel, controlling the amount of hydrogen pickup in processing,
developing alloys with improved resistance to hydrogen embrittlement, developing low or no
embrittlement plating or coating processes, and restricting the amount of in-situ (in position)
hydrogen introduced during the service life of a part.
Hydrogen embrittlement is a problem with zirconium and zirconium alloys, which often are used
as cladding materials for nuclear reactors. Zirconium reacts with water as follows.
Zr + 2 H2O ZrO2 + 2H2
Part of the hydrogen produced by the corrosion of zirconium in water combines with the
zirconium to form a separate phase of zirconium hydride (ZrH1.5) platelets. The metal then
becomes embrittled (ductility decreases) and it fractures easily. Cracks begin to form in the
zirconium hydride platelets and are propagated through the metal. Zircaloy-2 (a zirconium alloy),
which has been used as a fuel rod cladding, may absorb as much as 50% of the corrosion-
produced hydrogen and is subject to hydrogen embrittlement, especially in the vicinity of the
surface. Studies at Westinghouse, Batelle, and elsewhere have revealed that the nickel in the
zircaloy-2 was responsible for the hydrogen pickup. This has led to the development of zircaloy-
4, which has significantly less nickel than zircaloy-2 and is less susceptible to embrittlement. In
addition, the introduction of niobium into zircaloy-4 further reduces the amount of hydrogen
absorption.
MS-02
Page 38
Rev. 0