DOE-HDBK-1017/2-93
Plant Materials
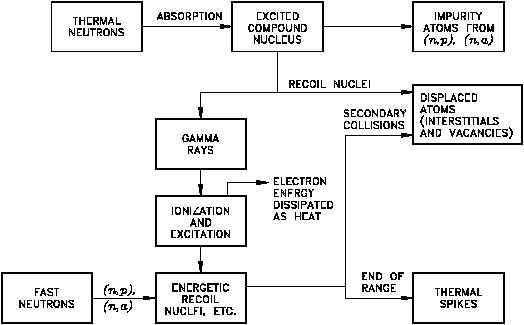
ATOMIC DISPLACEMENT DUE TO IRRADIATION
Atomic Displacements
If a target or struck nucleus gains about 25 eV of kinetic energy (25 eV to 30 eV for most
metals) in a collision with a radiation particle (usually a fast neutron), the nucleus will be
displaced from its equilibrium position in the crystal lattice, as shown in Figure 3.
The target nucleus (or recoiling atom) that is displaced is called a knocked-on nucleus or just a
Figure 3 Thermal and Fast Neutrons Interactions with a Solid
knock-on (or primary knock-on). When a metal atom is ejected from its crystal lattice the
vacated site is called a vacancy. The amount of energy required to displace an atom is called
displacement energy. The ejected atom will travel through the lattice causing ionization and
heating. If the energy of the knock-on atom is large enough, it may in turn produce additional
collisions and knock-ons. These knock-ons are referred to as secondary knock-ons. The process
will continue until the displaced atom does not have sufficient energy to eject another atom from
the crystal lattice. Therefore, a cascade of knock-on atoms will develop from the initial
interaction of a high energy radiation particle with an atom in a solid.
This effect is especially important when the knock-on atom (or nucleus) is produced as the result
of an elastic collision with a fast neutron (or other energetic heavy particle). The energy of the
primary knock-on can then be quite high, and the cascade may be extensive. A single fast
neutron in the greater than or equal to 1 MeV range can displace a few thousand atoms. Most
Rev. 0
Page 33
MS-05