Basic Electrical Theory
ATOM AND ITS FORCES
The strength of the attraction or of the repulsion force depends upon two factors: (1) the amount
of charge on each object, and (2) the distance between the objects. The greater the charge on
the objects, the greater the electrostatic field. The greater the distance between the objects, the
weaker the electrostatic field between them, and vice versa. This leads us to the law of
electrostatic attraction, commonly referred to as Coulomb’s Law of electrostatic charges, which
states that the force of electrostatic attraction, or repulsion, is directly proportional to the product
of the two charges and inversely proportional to the square of the distance between them as
shown in Equation 1-1.
(1-1)
F
K
q1
q2
d2
where
F
= force of electrostatic attraction or prepulsion (Newtons)
K
= constant of proportionality (Coulomb2/N-m2)
q1
= charge of first particle (Coulombs)
q2
= charge of second particle (Coulombs)
d
= distance between two particles (Meters)
If q1 and q2 are both either
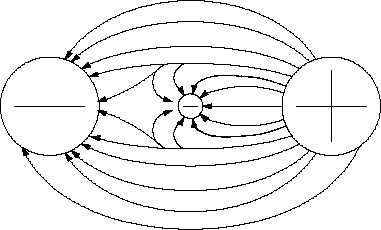
Figure 7 Potential Difference Between Two Charged Objects
positively
or
negatively
charged, the force is repulsive.
If q1 and q2 are opposite
polarity or charge, the force is
attractive.
Potential Difference
Potential difference is the term
used to describe how large the
electrostatic force is between
two charged objects.
If a
charged
body
is
placed
between two objects with a
potential
difference,
the
charged body will try to move
in one direction, depending
upon the polarity of the object. If an electron is placed between a negatively-charged body and
a positively-charged body, the action due to the potential difference is to push the electron toward
the positively-charged object. The electron, being negatively charged, will be repelled from the
negatively-charged object and attracted by the positively-charged object, as shown in Figure 7.
Rev. 0
Page 5
ES-01