Basic Electrical Theory
ATOM AND ITS FORCES
ATOM AND ITS FORCES
What is electricity? Electricity is defined as "the flow of electrons through simple
materials and devices" or "that force which moves electrons." Scientists think
electricity is produced by very tiny particles called electrons and protons. These
particles are too small to be seen, but exist as subatomic particles in the atom.
To understand how they exist, you must first understand the structure of the atom.
EO 1.1
DESCRIBE the following terms:
a.
Electrostatic force
b.
Electrostatic field
c.
Potential difference
d.
Electromotive force (EMF)
e.
Ion charge
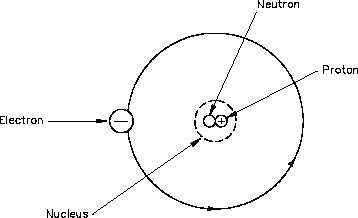
The Atom
Elements are the basic building
Figure 1 The Atom
blocks of all matter. The atom is
the smallest particle to which an
element can be reduced while still
keeping the properties of that
element. An atom consists of a
positively
charged
nucleus
surrounded by negatively charged
electrons, so that the atom as a
whole is electrically neutral. The
nucleus is composed of two kinds
of subatomic particles, protons and
neutrons, as shown in Figure 1.
The proton carries a single unit
positive charge equal in magnitude
to the electron charge.
The
neutron is slighty heavier than the
proton and is electrically neutral,
as the name implies. These two
particles exist in various combinations, depending upon the element involved. The electron is
the fundamental negative charge (-) of electricity and revolves around the nucleus, or center, of
the atom in concentric orbits, or shells.
Rev. 0
Page 1
ES-01