CHANGE OF PHASE
Thermodynamics
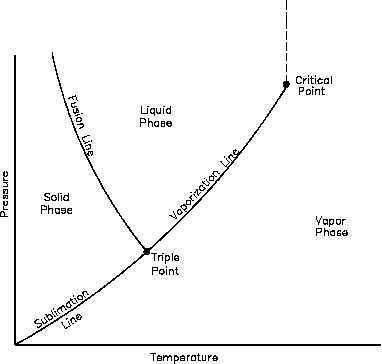
Figure 8 Pressure-Temperature Diagram
Condensation
All the processes discussed on the preceding pages (vaporization, sublimation, and fusion) occur
during a heat addition to a substance. If heat is removed from a substance, the opposite of the
described processes will occur.
As previously described, a heat addition at a constant pressure to a saturated liquid will cause
the liquid to evaporate (change phase from liquid to vapor). If heat is removed at a constant
pressure from a saturated vapor, condensation will occur and the vapor will change phase to
liquid. So the processes of vaporization and condensation are the exact opposite of each other.
Similarly, freezing is the opposite process of melting and fusion. Sublimation also has an
opposite process in which a gas goes directly to solid, but this process is not normally referred
to with a unique term.
Summary
The important information from this chapter is summarized on the following page.
HT-01
Page 38
Rev. 0