2R SO3 Na
Ca
HCO3
2R SO3Ca
2Na
HCO3
(resin complex)
(resin complex)
DOE-HDBK-1015/2-93
DISSOLVED GASES, SUSPENDED SOLIDS, AND pH CONTROL
Principles of Water Treatment
CH-04
Rev. 0
Page 16
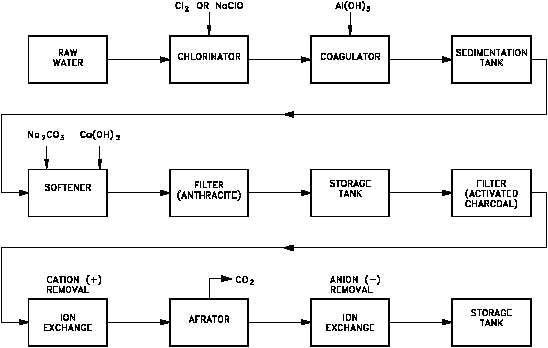
Figure 6 A Typical Pretreatment System
The reactions that occur in the water softener include the removal of both Mg and Ca ions.
++
++
The water softener contains resin in which the insoluble exchange site is the SO molecule, and
3
-
the soluble ions attached to the exchange site are Na ions. When water containing Mg , Ca ,
+
++
++
and HCO ions is passed over the resin in the softener, the ions are exchanged by the following
3
-
reaction (Mg removal is similar).
++
(4-6)
Note that electrical neutrality is maintained before and after the exchange reaction. One calcium
ion with two positive charges is attached to two exchange sites that release two sodium ions with
one positive charge each. The HCO ion is not affected by the reaction and passes through the
3
-
resin of the softener.
To obtain pure water, it is necessary to demineralize the water completely, which is accomplished
using a cation exchanger, an aerator, and an anion exchanger.