H R
Na
Na R
H
R OH
Cl
R Cl
OH
Principles of Water Treatment
DOE-HDBK-1015/2-93
WATER TREATMENT PROCESSES
Rev. 0
CH-04
Page 11
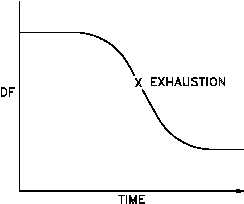
Figure 4 Typical History Curve
Resin performance may be monitored using a history curve that plots DF with respect to time.
A typical history curve is shown in Figure 4, with the resin considered "exhausted" at point X.
Specific Ion Exchanger Reactions
Suppose a solution containing Na ions is passed through hydrogen resin. From the relative
+
affinities given earlier, Na ions are attracted to the resin more strongly than H ions. Thus, Na
+
+
+
ions will displace H ions from the resin or, in other words, Na ions and H ions exchange
+
+
+
place between resin and solution. The process can be described by the following equilibrium
reaction.
(4-1)
In most practical situations, a solution containing impurities at low concentrations is passed
through a large amount of resin. By LeChatelier's Principle, the equilibrium of Reaction (4-1)
is forced far to the right. The equilibrium is displaced so far that, for practical purposes, all Na+
ions are removed from solution and replaced by H ions. As a result, the solution will be acidic
+
because of the excess of H ions.
+
If a solution containing Cl ions is passed through hydroxyl resin, the Cl ions will be removed
-
-
according to the following reaction.
(4-2)
Again, for a dilute solution and a large amount of resin, the removal of Cl ions is essentially
-
100 percent complete. In this case, the final solution will be basic because of the excess of
OH ions.
-