Atomic and Nuclear Physics
DOE-HDBK-1019/1-93
ATOMIC NATURE OF MATTER
Bohr Model of the Atom
The British physicist Ernest Rutherford postulated that the positive charge in an atom is
concentrated in a small region called a nucleus at the center of the atom with electrons existing
in orbits around it. Niels Bohr, coupling Rutherford's postulation with the quantum theory
introduced by Max Planck, proposed that the atom consists of a dense nucleus of protons
surrounded by electrons traveling in discrete orbits at fixed distances from the nucleus. An
electron in one of these orbits or shells has a specific or discrete quantity of energy (quantum).
When an electron moves from one allowed orbit to another allowed orbit, the energy difference
between the two states is emitted or absorbed in the form of a single quantum of radiant energy
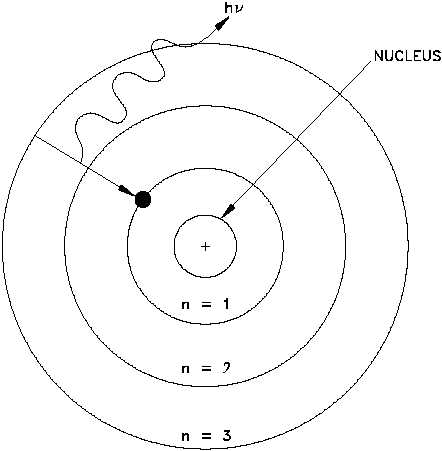
called a photon. Figure 1 is Bohr's model of the hydrogen atom showing an electron as having
just dropped from the third shell to the first shell with the emission of a photon that has an
energy = hv. (h = Planck's constant = 6.63 x 10-34 J-s and v = frequency of the photon.) Bohr's
theory was the first to successfully account for the discrete energy levels of this radiation as
measured in the laboratory. Although Bohr's atomic model is designed specifically to explain
the hydrogen atom, his theories apply generally to the structure of all atoms. Additional
information on electron shell theory can be found in the Chemistry Fundamentals Handbook.
Figure 1 Bohr's Model of the Hydrogen Atom
Rev. 0
Page 3
NP-01