Fundamentals of Chemistry
DOE-HDBK-1015/1-93
CHEMICAL BONDING
Rev. 0
CH-01
Page 29
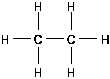
Figure 11 Alkane
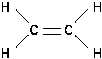
Figure 12 Alkene
The general formula for alkanes is C H
. The alkanes are
n
2n+2
colorless, practically odorless, insoluble in water, and readily
soluble in nonpolar solvents such as benzene or ether.
Alkanes are low in reactivity. The reactions they do undergo
are called halogenation, thermal decomposition (cracking),
and combustion. These are summarized below.
Halogenation occurs when a hydrogen atom is replaced
with a halogen atom. This is referred to as a
substitution reaction. There is no limit to how many
hydrogen atoms can be replaced in one molecule.
Thermal decomposition or cracking is the process of breaking large molecules into
smaller ones. Using heat as a catalyst, propane can be broken into methane and
ethylene:
Combustion occurs when an alkane is burned, the products being carbon dioxide gas,
water, and heat. These reactions are highly exothermic and as such the hydrocarbons
are frequently used for fuel.
Alkenes
Alkenes are hydrocarbons containing two fewer hydrogen
atoms than the corresponding alkane. The general formula for
alkenes is C H . These molecules will have a double bond as
n
2n
illustrated in Figure 12.
Because there are fewer hydrogen atoms than the maximum
possible, alkenes are unsaturated hydrocarbons. The chief
source for alkenes is the cracking of alkanes.