CHEMICAL BONDING
DOE-HDBK-1015/1-93
Fundamentals of Chemistry
CH-01
Rev. 0
Page 30
Figure 13 Alkyne
Figure 14 Aromatic
Figure 15 Alcohol
Alkynes
The third of the aliphatic hydrocarbons are the alkynes. These
compounds are unsaturated like the alkenes. They contain two
fewer hydrogens than the corresponding alkane, C H
. The
n
2n-2
alkyne hydrocarbons contain a triple bond between at least one
set of carbon atoms as illustrated in Figure 13.
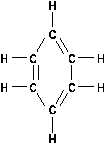
Aromatics
The other broad class of hydrocarbons is the aromatic
hydrocarbon. Rather than being arranged in straight chains, as
the aliphatics are, these are cyclic formations such as in
benzene. The derivatives of cyclic hydrocarbons have pleasant
(sometimes toxic) odors. The benzene in rubber cement is a
familiar odor. The cyclic compounds have alternating single -
double bonds as illustrated in Figure 14.
Aromatic hydrocarbons are very stable chemically, and act
very much like alkanes. They will undergo substitution
reactions rather than additions.
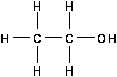
Alcohols
Alcohol is an aliphatic hydrocarbon with a hydroxyl (OH)
group substituting for one or more hydrogens as illustrated in
Figure 15.
The -OH functional group does not behave in an ionic manner
in the case of alcohols. The alcohols are molecular, not ionic,
in nature. Alcohols are versatile compounds which are often
used to make nearly every other kind of aliphatic compound.