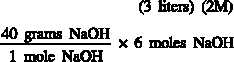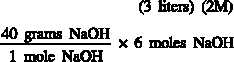?
CHEMICAL EQUATIONS
DOE-HDBK-1015/1-93
Fundamentals of Chemistry
CH-01
Rev. 0
Page 38
Example 2:
Prepare 3 liters of a 2M NaOH solution.
Solution:
Calculate the amount of NaOH required to prepare the solution.
a)
b)
substituting:
Therefore, to prepare 3 liters of a 2M NaOH solution, 240 grams of NaOH must be
weighed out and dissolved in water to make a volume of exactly 3 liters.
Normality
The normal concentration is another method for expressing the concentration of solutions.
Normality (N) is defined as the number of equivalents of solute dissolved in one liter of
solution.
One equivalent of acid is the amount of acid necessary to give up one mole of hydrogen ions
in a chemical reaction. One equivalent of base is the amount of base that reacts with one mole
of hydrogen ions. When expressing the concentrations of bases, normality refers to the number
of available hydroxyl ions. Because hydrogen and hydroxyl ions combine on a one-to-one basis,
one OH is equivalent to one H ion.
-
+
a)
H Cl
+
Na OH
Na Cl
+
H OH
+
-
+
-
+
-
+
-
Acid
Base
Salt
Water
b)
H SO
+
2K OH
K SO
+
2H OH
2
4
2
4
+
-
+
-
Acid
Base
Salt
Water