Fundamentals of Chemistry
DOE-HDBK-1015/1-93
CHEMICAL EQUATIONS
Rev. 0
CH-01
Page 39
Notice that in reaction a), one mole of HCl yields one equivalent per mole or one mole of H+
ions. H SO has two equivalents per mole or two H ions because each mole of the compound
2
4
+
can release two moles of hydrogen ions.
The number of equivalents of an acid or base can be determined from equivalent weight. The
equivalent weight is defined as the molecular weight of the acid or base divided by the number
of replaceable hydrogen or hydroxyl ions.
Example:
The equivalent weight of H SO is:
2
4
.
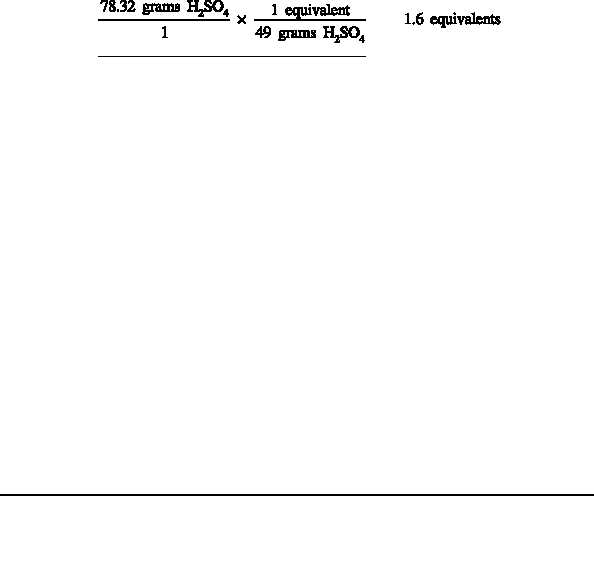
This means 49 grams of H SO is equal to one equivalent of H SO . If there is a one
2
4
2
4
liter solution that contains 78.32 grams H SO , the number of equivalents is:
2
4
.
Since normality is equal to the number of equivalents per liter, the normality of this
solution is 1.6 equivalents/liter, or 1.6 N.
Parts per Million
Another term used to describe the specific concentration of a solution is parts per million or
ppm. The term ppm is defined as the concentration of a solution in units of one part of solute
to one million parts solvent. One ppm equals one milligram of solute per liter of solution.
Another term, parts per billion (ppb), is defined as one part solute per one billion parts solvent.
One ppb is equal to one microgram solute per liter of solution. These two terms are usually
used for very dilute solutions.