Thermodynamics
SECOND LAW OF THERMODYNAMICS
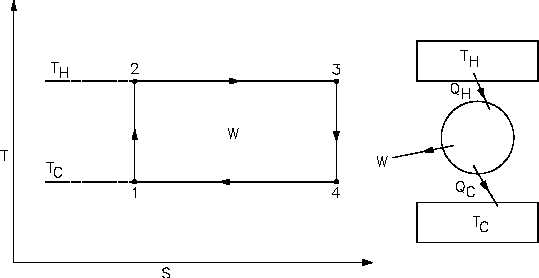
This cycle is known as a Carnot Cycle. The heat input (QH) in a Carnot Cycle is graphically
represented on Figure 21 as the area under line 2-3. The heat rejected (QC) is graphically
represented as the area under line 1-4. The difference between the heat added and the heat
rejected is the net work (sum of all work processes), which is represented as the area of rectangle
1-2-3-4.
Figure 21 Carnot Cycle Representation
The efficiency (h) of the cycle is the ratio of the net work of the cycle to the heat input to the
cycle. This ratio can be expressed by the following equation.
h
=
(QH - QC)/QH = (TH - TC)/TH
=
1 - (TC/TH)
(1-23)
where:
h
=
cycle efficiency
TC
=
designates the low-temperature reservoir (°R)
TH
=
designates the high-temperature reservoir (°R)
Rev. 0
Page 73
HT-01