Thermodynamics
SECOND LAW OF THERMODYNAMICS
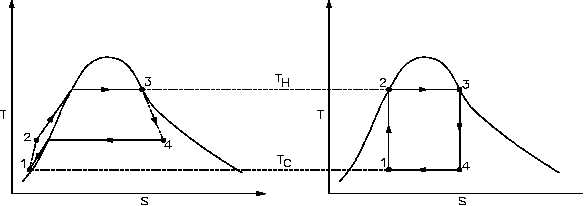
Figure 22 Real Process Cycle Compared to Carnot Cycle
Solution:
h
=
1 - (Tc / Th)
h
=
1 - (60 + 460)/(340 + 460)
=
1 - 520/800
=
35%
as compared to 18.0% actual efficiency.
An open system analysis was performed using the First Law of Thermodynamics in the previous
chapter. The second law problems are treated in much the same manner; that is, an isolated,
closed, or open system is used in the analysis depending upon the types of energy that cross the
boundary. As with the first law, the open system analysis using the second law equations is the
more general case, with the closed and isolated systems being "special" cases of the open system.
The solution to second law problems is very similar to the approach used in the first law analysis.
Figure 23 illustrates the control volume from the viewpoint of the second law. In this diagram,
the fluid moves through the control volume from section in to section out while work is delivered
external to the control volume. We assume that the boundary of the control volume is at some
environmental temperature and that all of the heat transfer (Q) occurs at this boundary. We have
already noted that entropy is a property, so it may be transported with the flow of the fluid into
and out of the control volume, just like enthalpy or internal energy. The entropy flow into the
control volume resulting from mass transport is, therefore,
insin, and the entropy flow out of the
m
control volume is
outsout, assuming that the properties are uniform at
m
Rev. 0
Page 75
HT-01