SECOND LAW OF THERMODYNAMICS
Thermodynamics
Early thermodynamic developments were centered around improving the performance of
contemporary steam engines. It was desirable to construct a cycle that was as close to being
reversible as possible and would better lend itself to the characteristics of steam and process
control than the Carnot cycle did. Towards this end, the Rankine cycle was developed.
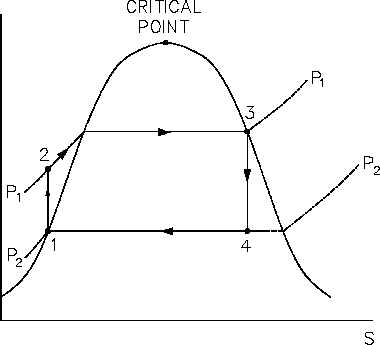
The main feature of the Rankine cycle, shown in Figure 31, is that it confines the isentropic
compression process to the liquid phase only (Figure 31 points 1 to 2). This minimizes the
amount of work required to attain operating pressures and avoids the mechanical problems
associated with pumping a two-phase mixture. The compression process shown in figure 31
between points 1 and 2 is greatly exaggerated*. In reality, a temperature rise of only 1°F occurs
in compressing water from 14.7 psig at a saturation temperature of 212°F to 1000 psig.
Figure 31 Rankine Cycle
* The constant pressure lines converge rapidly in the subcooled or compressed
liquid region and it is difficult to distinguish them from the saturated liquid line
without artificially expanding them away from it.
In a Rankine cycle available and unavailable energy on a T-s diagram, like a T-s diagram of a
Carnot cycle, is represented by the areas under the curves. The larger the unavailable energy,
the less efficient the cycle.
HT-01
Page 88
Rev. 0