H3O
+ e
H + H2O
CRUD AND GALVANIC CORROSION
DOE-HDBK-1015/1-93
Corrosion
CH-02
Rev. 0
Page 24
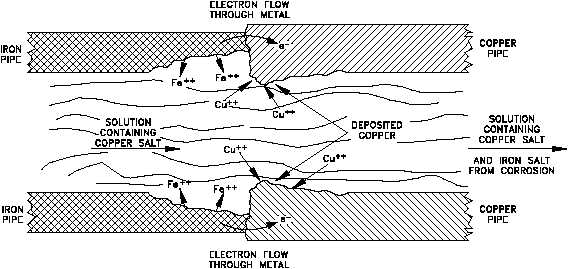
Figure 10 Galvanic Corrosion at Iron-Copper Pipe Junction
Figure 10 illustrates that galvanic corrosion occurs when two different metals are in contact and
exposed to an electrolyte.
Figure 10 shows the junction of iron and copper pipes containing a solution of a copper salt. The
oxidation potential of iron is sufficiently greater than that of copper so that iron is capable of
reducing Cu ions to copper metal. In this case, iron corrodes near the junction, and additional
+2
copper builds up on the copper pipe near the junction.
The solution to which the metal junction is exposed need not contain a salt of one of the metals
for galvanic corrosion to occur. If the iron-copper junction were exposed to water without Cu+2
ions, the reduction reaction would be as shown in Equation (2-4).
(2-4)
Again, iron would corrode near the junction, but in this case hydrogen would be formed on the
surface of the copper.
Prevention of Galvanic Corrosion
A method called cathodic protection, discussed previously in this module, is often used to retard
or eliminate galvanic corrosion. One of several ways of accomplishing this is to attach a third
metal to the metals to be protected. This metal must have an oxidation potential even greater
than that of the metal to be protected. The most active metal then tends to corrode in place of
the protected metal. The metal that corrodes to protect another metal is called a sacrificial anode.
This method is applied in the original design of structural materials. Zinc is a common sacrificial
anode and is often used in cooling water systems that contain seawater.