SPECIALIZED CORROSION
DOE-HDBK-1015/1-93
Corrosion
CH-02
Rev. 0
Page 28
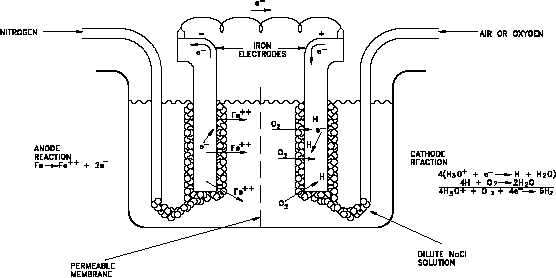
Figure 11 Differential Aeration Cell
Pitting corrosion occurs where the anodic site becomes fixed in a small area and the formation
of holes (deep attack) in an otherwise unaffected area takes place. Crevice corrosion is a type
of pitting corrosion that occurs specifically within the low flow region of a crevice.
To illustrate pitting attack, consider a special type of galvanic cell called a differential aeration
cell such as the one illustrated in Figure 11. This particular differential aeration cell is showing
current flow as a result of depolarization of one electrode (cathode) by oxygen. In this type of
cell, two iron electrodes are exposed to a dilute solution of an electrolyte (NaCl, for example).
Air (or oxygen) is bubbled around one electrode, and nitrogen is bubbled around the other. A
current flows through the wire connecting the two electrodes. The difference in potential is a
result of the difference in oxygen concentration at the two electrode surfaces. At the electrode
exposed to nitrogen, electrons are given up by the iron as it is oxidized. These electrons readily
flow through the external circuit to the electrode exposed to oxygen. At this depolarized
electrode they can participate in a reduction reaction. As a result, oxidation occurs at the
electrode exposed to nitrogen and reduction occurs at the aerated electrode. Oxidation at one
electrode and reduction at the other creates a potential and a flow of current through the
connecting wire. Note that loss of metal occurs at the electrode that is deficient in oxygen.