Corrosion
DOE-HDBK-1015/1-93
SPECIALIZED CORROSION
Rev. 0
CH-02
Page 29
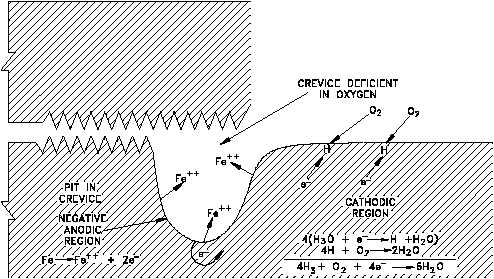
Figure 12 Representation of Crevice Pitting
In iron that is exposed to water, a similar action can occur if adjacent areas of the metal surface
become exposed to solutions with different oxygen concentrations. For example, the solution
in a crevice exchanges slowly with the bulk of the solution outside the crevice. Oxygen in the
solution inside the crevice will be depleted initially by the corrosion reaction.
(2-12)
This reaction alone does not produce a protective film on the metal. Because of restricted flow
into the crevice, replenishment of oxygen will be very slow; therefore, the solution inside the
crevice will have a low oxygen concentration relative to that outside the crevice as shown in
Figure 12. The two adjacent areas then establish a concentration cell with electrons flowing
from the region of low oxygen concentration to the region of high concentration. Thus, metal
goes into solution (oxidation) inside the crevice, and reduction occurs outside the crevice.
Metal ions diffuse out of the crevice, more metal dissolves, and the process continues. This
results in the formation of a pit inside the crevice.