SPECIALIZED CORROSION
DOE-HDBK-1015/1-93
Corrosion
CH-02
Rev. 0
Page 30
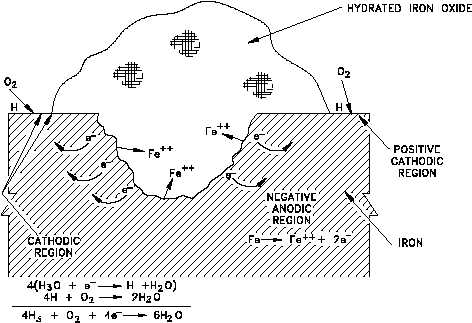
Figure 13 Pit in Metal Surface Promoted by Depolarization
The presence of oxygen can also promote pitting at areas on the metal surface that are initially
anodic with respect to an adjacent area. For example, suppose that adjacent areas on a metal
surface exhibit slightly different oxidation potentials. Oxidation, or loss of metal, proceeds at
the region of higher potential. Corrosion in the region of higher potential leads to formation
(at least initially) of a porous oxide film. The thickness of the film formed on the adjacent
cathodic region will be much less. Oxygen in the bulk of solution can reach the cathodic surface
(with the thin film) more readily than it can the nearby anodic surface region (with the thicker
oxide film). Depolarization of the cathodic region (thin film) by oxygen tends to maintain this
region cathodic, while a deficiency of oxygen under the thicker porous corrosion film assists
in maintaining an anodic condition in this region. The overall result is corrosion, or wasting
away, of the metal in the anodic region under the thicker film. Thus, a pit in the metal surface
is formed under the mound of surface oxide, as illustrated in Figure 13. Pitting of this type is
common in both low temperature and high temperature iron-water systems if precautions are
not taken to remove the oxygen from the water within the system.
It is also found that certain ions, notably chloride ions, cause pitting of iron and steel. The exact
mechanism by which this occurs is not clear, but in some way chloride ions cause defects in the
passivating oxide layer on the metal surface. The defects are highly localized and are
surrounded by large passive areas that tend to be cathodic. Thus, a small anodic (oxidation)
site is surrounded by a large cathodic (reduction) area. The current density will then be very
large at the anodic site, and attack on the metal will be rapid. In some test cases, deep pits have
been observed within a few hours.