Atomic and Nuclear Physics
DOE-HDBK-1019/1-93
ATOMIC NATURE OF MATTER
If only the electrostatic and gravitational forces existed in the nucleus, then it would be
impossible to have stable nuclei composed of protons and neutrons. The gravitational forces are
much too small to hold the nucleons together compared to the electrostatic forces repelling the
protons. Since stable atoms of neutrons and protons do exist, there must be another attractive
force acting within the nucleus. This force is called the nuclear force.
The nuclear force is a strong attractive force that is independent of charge. It acts equally only
between pairs of neutrons, pairs of protons, or a neutron and a proton. The nuclear force has a
very short range; it acts only over distances approximately equal to the diameter of the nucleus
(10
-13
cm). The attractive nuclear force between all nucleons drops off with distance much faster
than the repulsive electrostatic force between protons.
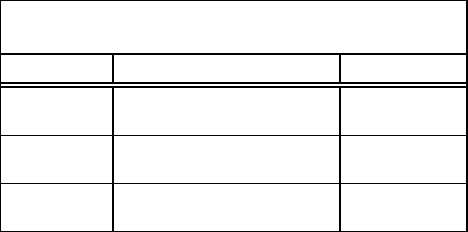
TABLE 3
Forces Acting in the Nucleus
Force
Interaction
Range
Gravitational
Very weak attractive force
between all nucleons
Relatively long
Electrostatic
Strong repulsive force between
like charged particles (protons)
Relatively long
Nuclear Force
Strong attractive force between
all nucleons
Extremely short
In stable atoms, the attractive and repulsive forces in the nucleus balance. If the forces do not
balance, the atom cannot be stable, and the nucleus will emit radiation in an attempt to achieve
a more stable configuration.
Summary
The important information in this chapter is summarized on the following page.
Rev. 0
Page 9
NP-01