Atomic and Nuclear Physics
DOE-HDBK-1019/1-93
RADIOACTIVITY
Rev. 0
Page 41
NP-01
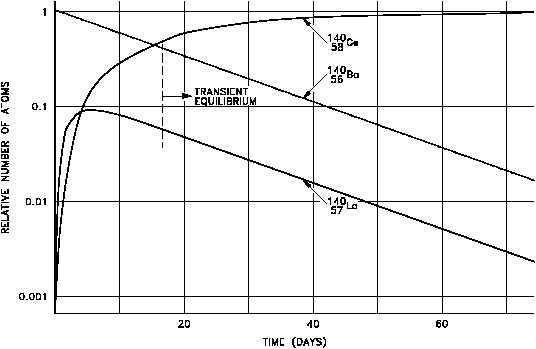
Figure 15 Transient Equilibrium in the Decay of Barium-140
The decay constant for barium-140 is considerably smaller than the decay constant for
lanthanum-140. Remember that the rate of decay of both the parent and daughter can be
represented as N. Although the decay constant for barium-140 is smaller, the actual rate of
decay ( N) is initially larger than that of lanthanum-140 because of the great difference in their
initial concentrations. As the concentration of the daughter increases, the rate of decay of the
daughter will approach and eventually match the decay rate of the parent. When this occurs,
they are said to be in transient equilibrium. A plot of the barium-lanthanum-cerium decay chain
reaching transient equilibrium is shown in Figure 15.
Secular equilibrium occurs when the parent has an extremely long half-life. In the long decay
chain for a naturally radioactive element, such as thorium-232, where all of the elements in the
chain are in secular equilibrium, each of the descendants has built up to an equilibrium amount
and all decay at the rate set by the original parent. The only exception is the final stable element
on the end of the chain. Its number of atoms is constantly increasing.
Summary
The important information in this chapter is summarized on the following page.