RADIOACTIVITY
DOE-HDBK-1019/1-93
Atomic and Nuclear Physics
NP-01
Page 38
Rev. 0
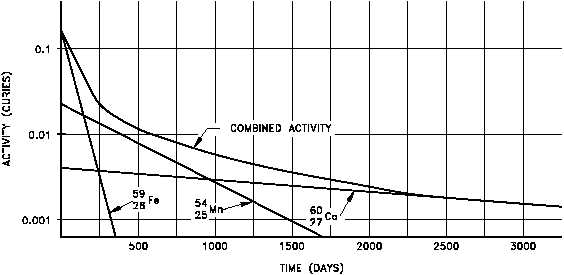
Figure 12 Combined Decay of Iron-56, Manganese-54, and Cobalt-60
Plotting the manner in which the activities of each of the three nuclides decay over time
demonstrates that initially the activity of the shortest-lived nuclide (iron-59) dominates the total
activity, then manganese-54 dominates. After almost all of the iron and manganese have
decayed away, the only contributor to activity will be the cobalt-60. A plot of this combined
decay is shown in Figure 12.
Radioactive Equilibrium
Radioactive equilibrium exists when a radioactive nuclide is decaying at the same rate at which
it is being produced. Since the production rate and decay rate are equal, the number of atoms
present remains constant over time.
An example of radioactive equilibrium is the concentration of sodium-24 in the coolant
circulating through a sodium-cooled nuclear reactor. Assume that the sodium-24 is being
produced at a rate of 1 x 10 atoms per second. If the sodium-24 were stable and did not decay,
6
the amount of sodium-24 present after some period of time could be calculated by multiplying
the production rate by the amount of time. Plotting the amount of material present would result
in the graph in Figure 13.
However, sodium-24 is not stable, and it decays with a half-life of 14.96 hours. If no
sodium-24 is present initially and production starts at a rate of 1 x 10 atoms per second, the rate
6
of decay will initially be zero because there is no sodium-24 present to decay. The rate of decay
of sodium-24 will increase as the amount of sodium-24 increases.