RADIOACTIVITY
DOE-HDBK-1019/1-93
Atomic and Nuclear Physics
NP-01
Page 40
Rev. 0
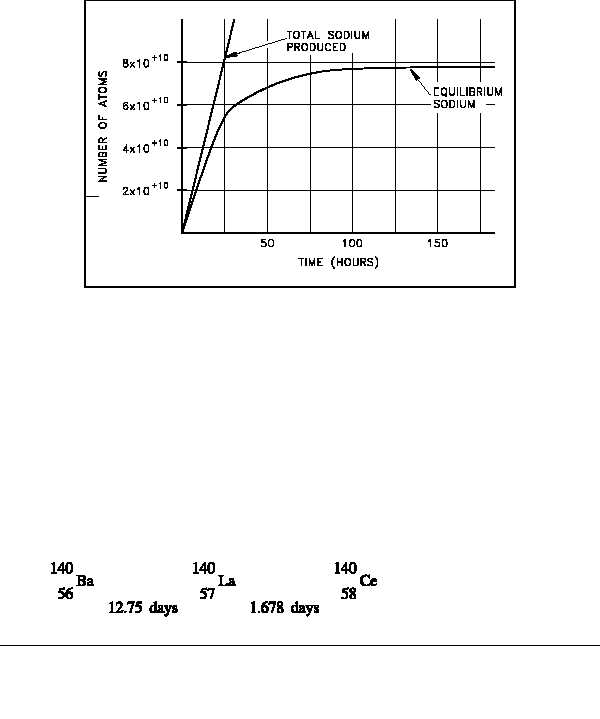
Figure 14 Approach of Sodium-24 to Equilibrium
The development of the equation to calculate how the amount of sodium-24 changes over time
as it approaches the equilibrium value is beyond the scope of this handbook. However, the
equation is presented below.
This equation can be used to calculate the values of the amount of sodium-24 present at different
times. As the time increases, the exponential term approaches zero, and the number of atoms
present will approach R/ . A plot of the approach of sodium-24 to equilibrium is shown in
Figure 14.
Transient Radioactive Equilibrium
Transient radioactive equilibrium occurs when the parent nuclide and the daughter nuclide
decay at essentially the same rate.
For transient equilibrium to occur, the parent must have a long half-life when compared to the
daughter. An example of this type of compound decay process is barium-140, which decays by
beta emission to lanthanum-140, which in turn decays by beta emission to stable cerium-140.