BATTERY THEORY
Batteries
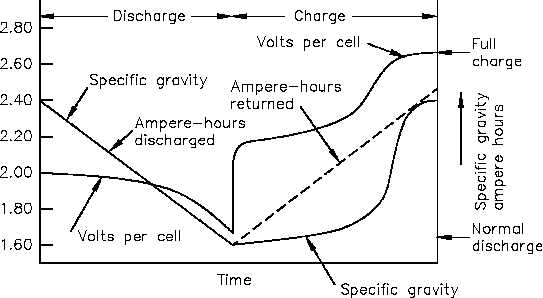
The decrease in specific gravity on discharge is proportional to the ampere-hours discharged.
While charging a lead-acid battery, the rise in specific gravity is not uniform, or proportional,
to the amount of ampere-hours charged (Figure 6).
The electrolyte in a lead-acid battery plays a direct role in the chemical reaction. The specific
Figure 6 Voltage and Specific Gravity During Charge and Discharge
gravity decreases as the battery discharges and increases to its normal, original value as it is
charged. Since specific gravity of a lead-acid battery decreases proportionally during discharge,
the value of specific gravity at any given time is an approximate indication of the battery’s state
of charge. To determine the state of charge, compare the specific gravity, as read using a
hydrometer, with the full charge value and the manufacturer’s published specific gravity drop,
which is the decrease from full to nominal charge value.
Example:
A lead-acid battery reads 1.175 specific gravity. Its average full charge specific
gravity is 1.260 and has a normal gravity drop of 120 points (or.120) at an 8 hour
discharge rate.
Solution:
Fully charged - 1.260
Present charge - 1.175
The battery is 85 points below its fully charged state. It is therefore about 85/120,
or 71%, discharged.
ES-04
Page 8
Rev. 0