Structure of Metals
DOE-HDBK-1017/1-93
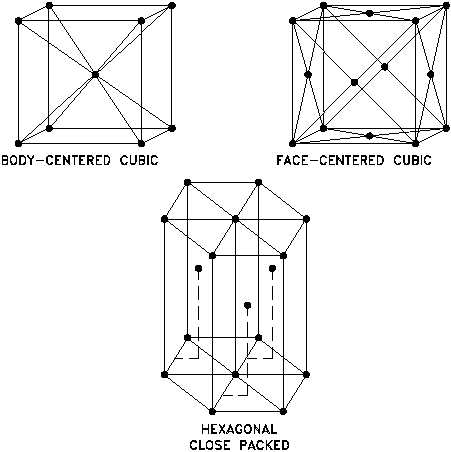
COMMON LATTICE TYPES
In a face-centered cubic (FCC) arrangement of atoms, the unit cell consists of eight atoms
at the corners of a cube and one atom at the center of each of the faces of the cube.
In a hexagonal close-packed (HCP) arrangement of atoms, the unit cell consists of three
layers of atoms. The top and bottom layers contain six atoms at the corners of a hexagon
and one atom at the center of each hexagon. The middle layer contains three atoms
nestled between the atoms of the top and bottom layers, hence, the name close-packed.
Figure 2 Common Lattice Types
Most diagrams of
the structural cells
for the BCC and
FCC forms of iron
are
drawn
as
though they are of
the same size, as
shown in Figure 2,
but they are not.
I n
t h e
B C C
arrangement,
the
structural
cell,
which uses only
nine
atoms,
is
much smaller.
Rev. 0
Page 7
MS-01