Fundamentals of Chemistry
DOE-HDBK-1015/1-93
CHEMICAL BONDING
Rev. 0
CH-01
Page 25
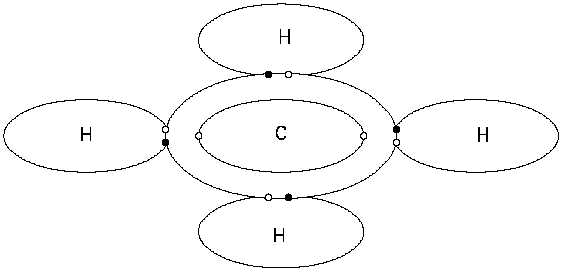
Figure 7 Covalent Bond, Methane CH4
Because of this, the positive and negative ions arrange themselves in three dimensions, as
shown in Figure 6(B), to balance the charges among several ions. In sodium chloride, for
example, each chloride ion is surrounded by as many sodium ions as can easily crowd around
it, namely six. Similarly, each sodium ion is surrounded by six chloride ions. Therefore, each
chloride ion is bonded to the six nearest sodium ions and bonded to a lesser extent to the more
distant sodium ions. Accordingly, the ionic bond is a force holding many atoms or ions
together rather than a bond between two individual atoms or ions.
Covalent Bonds
A covalent bond is formed when one or more electrons from an atom pair off with one or more
electrons from another atom and form overlapping electron shells in which both atoms share the
paired electrons. Unlike an ionic bond, a covalent bond holds together specific atoms. Covalent
bonding can be single covalent, double covalent, or triple covalent depending on the number of
pairs of electrons shared. Figure 7 shows the bonding that occurs in the methane molecule,
which consists of four single covalent bonds between one carbon atom and four hydrogen atoms.
Two double covalent bonds result when carbon dioxide, which consists of one carbon atom
and two oxygen atoms, is formed. Four pairs of electrons are shared by the carbon atom, two
from each of the two oxygen atoms as shown in Figure 8. A combination of two electrons
form a combination of lower energy than their energy when separated. This energy difference
represents the force that binds specific atoms together.