CHEMICAL BONDING
DOE-HDBK-1015/1-93
Fundamentals of Chemistry
CH-01
Rev. 0
Page 24
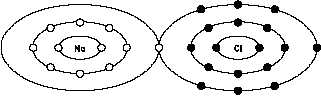
Figure 6 Ionic Bond, Sodium Chloride
In the case of H + Br , this is likely to take place because the exchange would satisfy the
+
-
needs of both atoms. Although there is far more to consider than just the number of valence
electrons, this is a good rule of thumb.
If the atom needed two electrons and only picked up one, it would still actively seek out an
additional electron. The reaction of H + Te is far less likely to take place because the
+
-2
resulting molecule would still have an incomplete valence shell. Of course, the combining of
two atoms, when both want to release or gain electrons, may take place (for example; H or
2
O ) but is less probable when other atoms are available.
2
Atoms are joined or bonded together through this interaction of their electrons. There are
several types of chemical bonds that hold atoms together; three will be discussed, ionic,
covalent, and metallic.
Ionic Bonds
An ionic bond is formed when one or more electrons is wholly transferred from one element
to another, and the elements are held together by the force of attraction due to the opposing
charges. An example of ionic bonding is shown in Figure 6(A) for sodium chloride (table salt).
The sodium atom loses the one electron in its outer shell to the chlorine atom, which uses the
electron to fill its outer shell. When this occurs, the sodium atom is left with a +1 charge and
the chlorine atom a -1 charge. The ionic bond is formed as a result of the attraction of the two
oppositely-charged particles. No single negatively-charged ion has a greater tendency to bond
to a particular positively-charged ion than to any other ion.