CHEMICAL BONDING
DOE-HDBK-1015/1-93
Fundamentals of Chemistry
CH-01
Rev. 0
Page 26
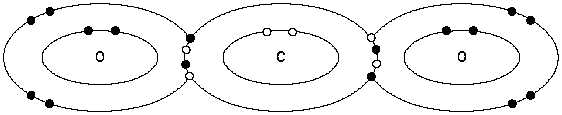
Figure 8 Formation of the Carbon Dioxide Molecule
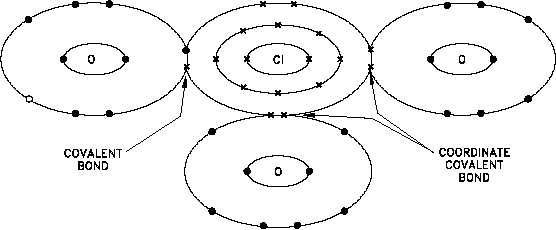
Figure 9 Coordinate Covalent Bond, Chlorate Ion ClO3
When both shared electrons in a covalent bond come from the same atom, the bond is called
a coordinate covalent bond. Although both shared electrons come from the same atom, a
coordinate covalent bond is a single bond similar in properties to a covalent bond. Figure 9
illustrates the bonds of the negatively-charged chlorate ion. The ion consists of one chlorine
atom and three oxygen atoms with a net charge of -1, and is formed with two coordinate
covalent bonds and one covalent bond. The chlorine atom has effectively gained an electron
through the covalent bond, which causes the overall negative charge.
Covalent bonds can be either polar or nonpolar. When the shared pair of electrons is not
shared equally, one end of the bond is positive, and the other end is negative. This produces
a bond with two poles called a polar covalent bond.