Corrosion
DOE-HDBK-1015/1-93
CORROSION THEORY
Rev. 0
CH-02
Page 3
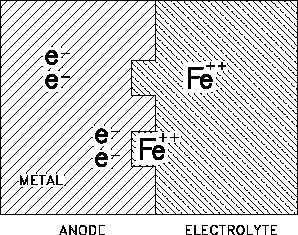
Figure 1 Formation of Ferrous (Fe ) Ions in
++
the Corrosion of Iron
Electrochemical Cells
Corrosion is electrochemical in nature because the corrosive chemical reactions involve transfer
of charge. Figure 1 shows the transfer of charge when iron is in contact with water or an acidic
water solution.
Iron goes into solution as Fe ions. As
++
these ions go into solution, the metal
becomes negatively charged (by the
electrons left behind) with respect to the
electrolyte. A potential difference (voltage)
is produced between the electrolyte and the
metal. The process in which electrons are
given up and positive metal ions are formed
is called oxidation. The sites at which the
oxidation takes place on the surface of the
metal become electrochemical cells made up
of micro-electrodes of the two different
substances; the metal and the electrolyte.
These micro-electrodes set up many
micro-cells connected through the bulk of
the metal. If a different metal is used, it will
go into solution to a greater (or lesser)
extent producing a larger (or smaller) potential difference between the metal and electrolyte than
was the case for iron. For example, magnesium and zinc go into solution to a greater extent than
iron, and these metals will be more negative with respect to the electrolyte than iron. Nickel,
lead, and copper go into solution less readily and produce a smaller potential difference. Table 1
lists the potential differences for various metals in water. The order of the series can change for
different electrolytes (for example, different pH, ions in solution).
Electrochemical cells and oxidation potentials are very important in understanding most
corrosion processes. Examples of electrochemical cells include galvanic cells (cells made up of
electrodes of two different substances) and concentration cells (cells containing electrodes of the
same substance under different conditions of concentration).