Corrosion
DOE-HDBK-1015/1-93
CORROSION THEORY
Rev. 0
CH-02
Page 5
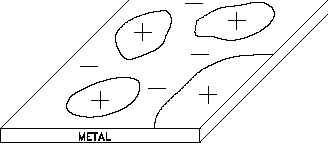
Figure 2 Metal Surface Showing Arrangement of Micro-cells
Consider iron in water again. If the
surface of the iron and the water
solution were uniform, iron would go
into solution as Fe ions until the
++
difference in potential between the
positively-charged solution and the
negatively-charged metal stopped the
iron ions from leaving the surface. In
practice, though, impurities and
imperfections (for example, oxide
coatings) lead to preferential removal
of metal from certain parts of the
surface, and potential differences arise as in the two metal system. The corrosion cells, changing
as surface and solution differences change, cause general overall corrosion. If the cells do not
shift, pitting results.
Oxidation-Reduction Reactions
The corrosion of a metal (that is, the chemical transformation that is recognized as destructive
to the metal) is the oxidation step of the overall oxidation-reduction process. Oxidation is the
process of losing electrons; reduction is the process of gaining electrons. The metal atoms
release electrons (are oxidized) and become positive ions. The site at which this occurs is known
as the anode. Typical oxidation half-reactions include the following.
(2-1)
(2-2)
(2-3)
The cations (positive ions) may then go into solution, or they may combine with any available
anions (negative ions) or water to form ionic compounds. The exact fate of the cations is
important to subsequent processes, but the primary effect is that atoms leave the metallic state,
and the metal deteriorates.
An oxidation process cannot take place without a simultaneous reduction (gain of electrons)
process. The nature of the reduction step in corrosion sometimes varies with the metal and the
environment to which it is exposed. For most metals in an aqueous environment, the important
reduction half-reaction is the reduction of hydronium ions (a hydronium ion is simply a hydrogen
ion attached to a water molecule).
(2-4)