CORROSION THEORY
DOE-HDBK-1015/1-93
Corrosion
CH-02
Rev. 0
Page 8
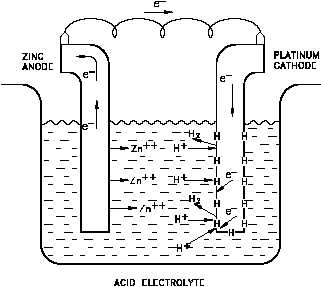
Figure 4 A Galvanic Cell Showing Absorbed Hydrogen
Atoms on a Cathode
Now consider a galvanic cell with zinc
and platinum electrodes, such as that
shown in Figure 4. The half-reactions
in the cell are as follows.
(2-4)
Again, as the cell operates, the cell
potential drops. The decrease is
partially due to the increase in Zn+2
concentration and the decrease in H O
3
+
concentration, but another type of
polarization also occurs in this cell.
This second type is associated with the
reduction half-reaction.
The hydrogen atoms formed by the
reaction of Equation (2-4) absorb on
the surface of the metal and remain
there until removed by one of two processes: combination of two hydrogen atoms to form
molecular hydrogen, which is then released as a gas or reaction with dissolved oxygen to form
water. In the absence of oxygen (deaerated solutions), the first process applies.
(2-6)
Combining Equation (2-6) with Equation (2-4), the net reduction half-reaction is obtained.
(2-6)
(2-7)
Until the absorbed hydrogen atoms are removed from the metal surface, they effectively block
the sites at which the reaction of Equation (2-4) can occur. At low temperatures the reaction
of Equation (2-6) is slow relative to the reaction of Equation (2-4) because, although the
reaction is energetically favored, the combination of two hydrogen atoms requires a large
activation energy. Equation (2-6) shows the rate-controlling step of the net reduction
half-reaction. Because the oxidation half-reaction can occur no faster than the reduction
half-reaction, the rate of the overall oxidation-reduction reaction is controlled by the reaction
of Equation (2-6).