Corrosion
DOE-HDBK-1015/1-93
CORROSION THEORY
Rev. 0
CH-02
Page 7
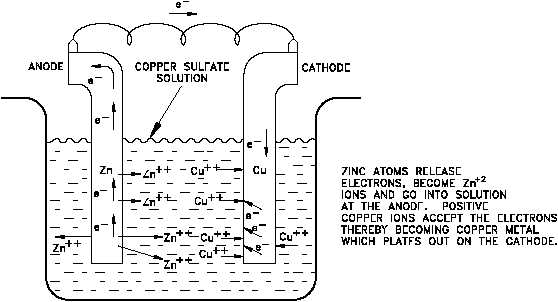
Figure 3 A Galvanic Cell
An active electrochemical cell (oxidation-reduction reaction) is an unstable chemical system.
The potential associated with a galvanic cell, for example, steadily decreases as current flows and
the oxidation-reduction reaction proceeds. Eventually, the potential falls to zero, the cell no
longer supplies electrical energy, and no further net reaction takes place. At this point the
system is at equilibrium. In electrochemical cells, the decrease in cell potential caused by the
operation of the cell (current flow) is called polarization.
This change in cell potential can be determined. Consider the zinc-copper galvanic cell shown
in Figure 3. As the reaction takes place, Zn ions (produced by the oxidation of zinc metal) pass
+2
into solution. The Cu ions in solution are reduced as the copper metal plates out. Thus, the
+2
concentration of Zn in solution increases and the concentration of Cu decreases according
+2
+2
to the following overall reaction.
(2-5)
As Zn increases and Cu decreases, the electrical potential decreases. This decrease in cell
+2
+2
potential, which results from changes in concentrations, is one form of polarization called
concentration polarization.