MASS DEFECT AND BINDING ENERGY
DOE-HDBK-1019/1-93
Atomic and Nuclear Physics
An atom cannot stay in the excited state for an indefinite period of time. An excited atom will
eventually transition to either a lower-energy excited state, or directly to its ground state, by
emitting a discrete bundle of electromagnetic energy called an x-ray. The energy of the x-ray
will be equal to the difference between the energy levels of the atom and will typically range
from several eV to 100,000 eV in magnitude.
Energy Levels of the Nucleus
The nucleons in the nucleus of an atom, like the electrons that circle the nucleus, exist in shells
that correspond to energy states. The energy shells of the nucleus are less defined and less
understood than those of the electrons. There is a state of lowest energy (the ground state) and
discrete possible excited states for a nucleus. Where the discrete energy states for the electrons
of an atom are measured in eV or keV, the energy levels of the nucleus are considerably greater
and typically measured in MeV.
A nucleus that is in the excited state will not remain at that energy level for an indefinite period.
Like the electrons in an excited atom, the nucleons in an excited nucleus will transition towards
their lowest energy configuration and in doing so emit a discrete bundle of electromagnetic
radiation called a gamma ray (g-ray). The only differences between x-rays and g-rays are their
energy levels and whether they are emitted from the electron shell or from the nucleus.
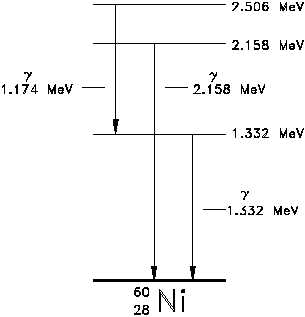
The ground state and the excited states of
Figure 7 Energy Level Diagram - Nickel-60
a nucleus can be depicted in a nuclear
energy-level diagram.
The nuclear
energy-level diagram consists of a stack of
horizontal bars, one bar for each of the
excited states of the nucleus. The vertical
distance between the bar representing an
excited state and the bar representing the
ground state is proportional to the energy
level of the excited state with respect to
the ground state.
This difference in
energy between the ground state and the
excited state is called the excitation energy
of the excited state. The ground state of
a nuclide has zero excitation energy. The
bars for the excited states are labeled with
their respective energy levels. Figure 7 is
the energy level diagram for nickel-60.
NP-01
Page 20
Rev. 0